Abstract
CMV recipient seropositivity (R+) and CMVi are independent risk factors for increased mortality after alloHCT. Preemptive therapy (PET) was standard of care until LTV approval by the FDA in November 2017 for CMVi prevention in CMV R+ alloHCT patients (pts). In a registration trial, LTV led to a significant reduction in clinically significant CMVi (CS-CMVi) defined as CMVi requiring PET in both high-risk (HR) or low-risk (LR) recipients. In the HR-group, defined as mismatched related / unrelated donor with at least one mismatch in one of the four HLA-gene loci of HLA-A, -B, -C or -DRB1, haploidentical donor, umbilical cord source or grade ≥2 acute graft-versus-host disease (aGVHD) at randomization, the impact of LTV on CS-CMVi was more robust. Small studies have confirmed the positive impact of LTV on CS-CMVi. Here, we compared the natural history of CMVi and CS-CMVi between the pre-LTV and LTV era in the first 100 days after HR-alloHCT. We also explored the impact on non-relapse mortality (NRM), overall survival (OS), disease free survival (DFS), and incidence of aGVHD between the two eras.
In this IRB approved retrospective study, we identified 450 consecutive HR-alloHCT pts who underwent their first HCT from 1/1/2016 to 12/31/2020 at our center. Pre-LTV era was from 1/1/2016 to 2/28/2018 and LTV era was from 3/1/2018 onwards when prophylaxis became standard of care (SOC) for all R+ alloHCT at our institution. In the HR-alloHCT, the uptake of the new SOC was consistent in all HR-R+ pts beginning LTV prophylaxis on day +7 post-HCT. We defined R+ HR-alloHCT pts at high-risk for CMVi or CS-CMVi as described above except for aGVHD (not recorded at time of institution of LTV). CMVi was defined as first time viral load (VL) of >500 genomic copies/ml (gc/ml). CS-CMVi was defined as a VL >500 gc/ml (910 IU/ml) on two consecutive tests done atleast 48 hours apart, that triggered PET (ganciclovir, valganciclovir, foscarnet, cidofovir), or had identification of CMV end organ disease . The incidence of CMVi and CS-CMVi in R+ allo-HCT was compared by LTV era using Gray test. Kaplan-Meier curves and log-rank tests were used for OS and DFS by LTV era. NRM, relapse, acute and chronic GVHD were compared using cumulative incidence curves and Gray test. All tests were 2-sided at 0.05 level.
Of the 450 HR-alloHCT pts, 146 were R+ in pre-LTV vs. 246 R+ in LTV era. R+ patient, their eligible underlying disease, and HCT characteristics are shown in Table 1. There was a significant reduction in both CMVi and CS-CMVi in LTV era vs pre-LTV era (24.1% vs 45.2%, and 22.3% vs 44.5% respectively; p <0.001 for both outcomes) in the first 100 days. Compared to pre LTV era, LTV era was associated with significantly reduced CS-CMVi among R+ pts (HR=0.39, 95%CI: 0.26-0.58, p <0.001) in the multivariable Fine and Gray model adjusted for primary diagnosis, donor type and acute GVHD. CMVi was also reduced in the multivariable model (HR=0.41 and 95%CI: 0.28-0.61, p<0.001).
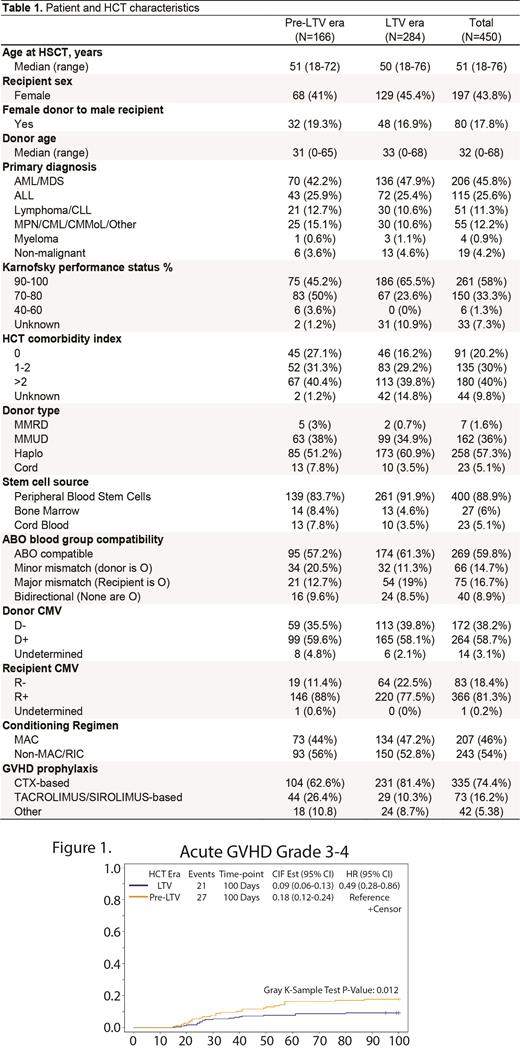
Although there were no significant differences in OS, DFS, NRM, relapse, and chronic GVHD between the two eras at 6, 12, and 18 months post-HCT in R+ pts, a trend towards improved OS and DFS in LTV era was noted (p=0.06 and p=0.07) in this patient population. There was a significantly lower rate of grade III-IV acute GVHD in the LTV era (9.2% vs 17.8% at day 100, p=0.012 with HR = 0.49). No case of CMV disease was identified in the first 100 days.
LTV has substantially reduced CS-CMVi in the first 100 days post-HCT in HR-R+ pts and resultant burden from PET. We identified a significant reduction in grade III - IV aGVHD in LTV era suggesting that with reduced CMVi, LTV may have a salutary impact on development of aGVHD; this is in agreement with studies showing bidirectional relationship between CMVi and onset of aGVHD. We did not observe a significant difference in OS, DFS, NRM amongst the two eras but there was trend towards higher OS and DFS in LTV era that requires further assessment in a larger multicenter cohort. Lastly, significant burden persists from CS-CMVi in this patient population during the first 100 days of alloHCT that underscores the need of efforts to identify other novel methods to mitigate it. One of the limitations in the LTV era is identifying the clinical scenarios surrounding the CMVi and CS-CMVi that may relate to compliance, absorption from gastrointestinal tract, and affordability or coverage of LTV after discharge from hospital.
Dadwal: Astellas: Speakers Bureau; Aseptiscope: Consultancy; AlloVir: Research Funding; Shire/Takeda: Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Other: Investigator; Karius: Other: Investigator. Marcucci: Novartis: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings. Taplitz: Merck: Membership on an entity's Board of Directors or advisory committees. Artz: Radiology Partners: Other: Spouse has equity interest in Radiology Partners, a private radiology physician practice. Stein: Amgen: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Stemline: Speakers Bureau. Forman: Allogene: Consultancy; Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company. Al Malki: Neximmune: Consultancy; Jazz Pharmaceuticals, Inc.: Consultancy; CareDx: Consultancy; Rigel Pharma: Consultancy; Hansa Biopharma: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal